FDA seeks probe into communications between staff and Biogen over Alzheimer’s drug
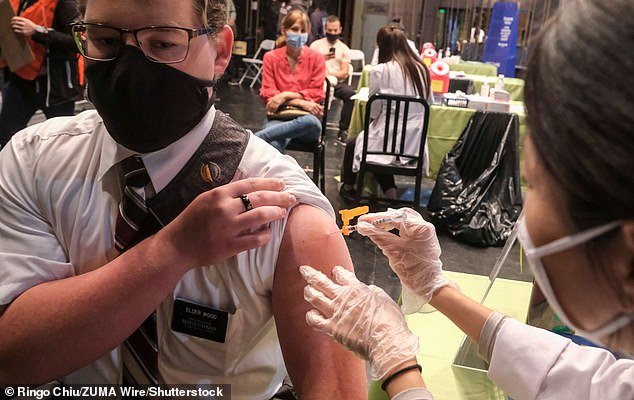
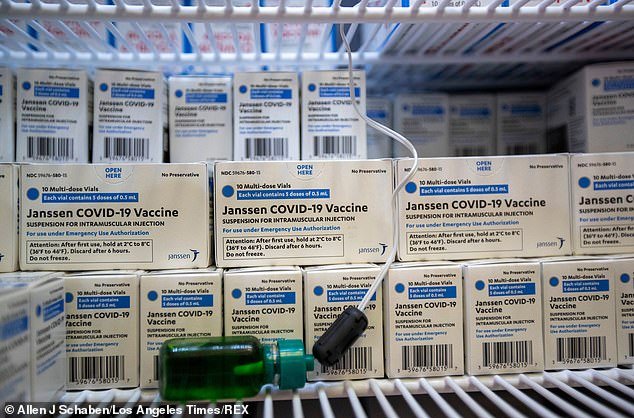
BREAKING NEWS: FDA seeks probe into communications between its staff and Biogen after controversial approval of Alzheimer’s drug FDA commissioner Janet Woodcock has asked for a probe into her staff’s communications with Biogen Biogen’s Aduhelm received FDA approval for Alzhiemer’s disease in early June despite limited data that it worked in clinical trials By Mansur Shaheen … Read more