Use of the AstraZeneca Covid-19 vaccine should be suspended following reports of serious post-jab blood clots in Norway, Ireland’s deputy medical chief said.
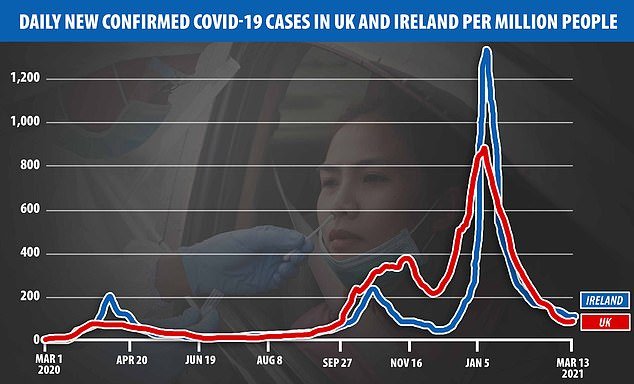
Irish authorities have been pushing the pharmaceutical giant to speed up its vaccine supplies to the Republic, where cases per million people exceeded the UK’s figures during the peak of the January wave.
But now, deputy chief medical officer Dr Ronan Glynn said Ireland will act on a ‘precautionary principle’ and pause the AstraZeneca rollout following reports of ‘serious blood clotting events’ in Norway.
It will join Denmark, Norway and Iceland in temporarily halting all AstraZeneca vaccinations to investigate the claims.
Norwegian health authorities confirmed that three healthcare workers who had the AstraZeneca jab were being treated in hospital for bleeding, blood clots and a low count of blood platelets.
Use of the AstraZeneca Covid-19 vaccine should be suspended following reports of serious post-jab blood clots in Norway, Ireland’s deputy medical chief said. Pictured: Daily confirmed Covid cases per million people in Ireland and the UK
Irish authorities have been pushing the pharmaceutical giant to speed up its jab supplies to the Republic after Boris Johnson made it clear that Britain would not send its vaccines to Ireland until people in the UK have had the jab. Pictured: A vaccine centre in Dublin

Deputy chief medical officer Dr Ronan Glynn said Ireland will act on a ‘precautionary principle’ and pause the AstraZeneca rollout following reports of ‘serious blood clotting events’ in Norway
Dr Glynn said: ‘This recommendation has been made following a report from the Norwegian Medicines Agency of four new reports of serious blood clotting events in adults after vaccination with Covid-19 vaccine AstraZeneca.
‘It has not been concluded that there is any link between the Covid-19 vaccine AstraZeneca and these cases.
‘However, acting on the precautionary principle, and pending receipt of further information, the National Immunisation Advisory Committee (NIAC) has recommended the temporary deferral of the Covid-19 vaccine AstraZeneca vaccination programme in Ireland.’
Norwegian health authorities confirmed that three healthcare workers who had the AstraZeneca jab (file image) were being treated in hospital for bleeding, blood clots and a low count of blood platelets
A nurse vaccinates a man with the AstraZeneca Covid-19 vaccine during a mass vaccination campaign for people between ages of 50 to 55 in Vigo, northwestern Spain
Norway halted the rollout of the AstraZeneca vaccine on Thursday, following a similar move by Denmark. Iceland later followed suit.
All three individuals in hospital in Norway for conditions including blood clots were under the age of 50. The Government were notified on Saturday.
Senior doctor at the Norwegian Medicines Agency Sigurd Hortemo told a news conference this week: ‘We do not know if the cases are linked to the vaccine.’
The European medicine regulator, the European Medicines Agency (EMA), would investigate the three incidents, Hortemo said.
Medical Director at the Norwegian Medicines Agency Steinar Madsen said: ‘They have very unusual symptoms: bleeding, blood clots and a low count of blood platelets.
‘They are quite sick […] We take this very seriously.’
Meanwhile, the European Medicines Agency (EMA) reported one person in Austria was diagnosed with blood clots and died 10 days after vaccination – but it stressed there is ‘currently no indication that vaccination has caused these conditions’.
A further patient was admitted to hospital in Austria with pulmonary embolism – a blockage in the arteries in the lungs – after being vaccinated, while one death involving a blood clot was reported in Denmark.
A 50-year-old man is also thought to have died in Italy from deep vein thrombosis (DVT), while there has been an unconfirmed report of another death in the country.
Italy also followed Austria, Estonia, Latvia, Luxembourg and Lithuania in banning jabs from one particular batch of one million AstraZeneca vaccines, which was sent to 17 countries, after reports of a death.
Ireland’s governing coalition has been under fire over the speed of its vaccination response.
AstraZeneca said an analysis of its safety data covering reported cases from over 17 million vaccine doses given had shown no evidence of an increased risk of pulmonary embolism, deep vein thrombosis or thrombocytopenia – having low levels of platelets.
‘In fact, the reported numbers of these types of events for COVID-19 Vaccine AstraZeneca are not greater than the number that would have occurred naturally in the unvaccinated population,’ a company spokeswoman said.
Such trends or patterns were also not observed during clinical trials for the vaccine, she added.
Around 600,000 doses of vaccine – across all manufacturers – have so far been delivered.
That includes the most elderly, those in nursing homes and healthcare workers.
AstraZeneca was approved for use early in the UK. Northern Ireland has made proportionately faster progress than the Republic in reaching the most vulnerable.
Irish Taoiseach Micheal Martin held ‘positive’ discussions with AstraZeneca chief executive officer Pascal Soriot on Friday evening.
He has expressed frustration at the failure of the company to meet delivery schedules for inoculations.
Ireland’s Minister of State for Mental Health and Older people has said Ireland ‘is still on track’ to have all over-70s vaccinated by mid-May with the supplies they have received to date, despite disruption from AstraZeneca.
Norway halted the rollout of the AstraZeneca vaccine (file image) on Thursday, following a similar move by Denmark. Iceland later followed suit