Regeneron to ask FDA to use its COVID-19 antibody drug as a preventative treatment
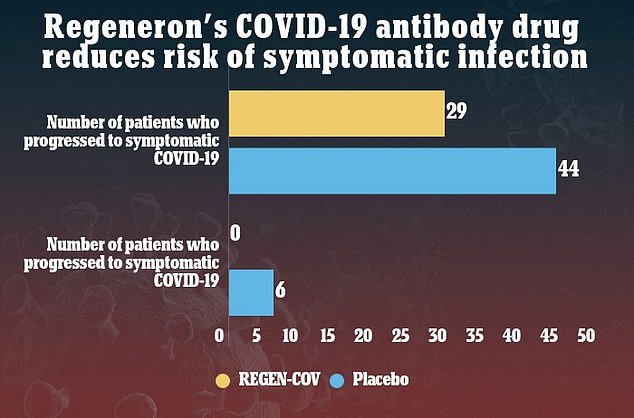
Regeneron Pharmaceuticals plans to ask the U.S. Food and Drug Administration (FDA) to allow its COVID-19 monoclonal antibody cocktail to be used as a preventative treatment. It comes after data from a Phase III clinical trial, run together with the National Institutes of Health, found the drug reduced the risk of asymptomatic coronavirus patients developing symptoms … Read more